Contents
Reaction mechanism
A reaction mechanism is the actual process by which a reaction takes place. It explains which bonds are broken, in what order, how many steps are involved, the relative rate of each step etc.
- The reaction mechanism describes the sequence of elementary reactions that must occur to go from reactants to products.
- Reaction intermediates are formed in one step and then consumed in a later step of the reaction mechanism.
Types of mechanism
Depending on how the bonds break, organic mechanisms can be divided into three basic types.
a. Homolytic or free radical mechanism :
If a bond breaks in such a way that each fragment gets one electron , free radicals are formed and such reactions are said to take place by hemolytic or free radical mechanism.

b. Heterolytic mechanism :
If a bond breaks in such a way that both bonding electrons remain with one fragment, ions are formed and such reactions are said to take place by heterolytic mechanism.

c. Pericyclic mechanism :
This mechanism involves the cyclic movements of the electrons in the bond breaking and bond making. There are no intermediates, ions or free radicals. Reactions with this type of mechanism are called pericyclic mechanism.

Thermodynamic and kinetic requirements for a reaction
Thermodynamic requirements for a chemical reaction :
For a reaction to take place spontaneously, the free energy of the products must be lower than the free energy of the reactants. i.e. ∆G must be negative.
We know that
∆G = ∆H – T∆S
Where,
∆G = Change in free energy
∆H = Change in enthalpy
∆S = Change in entropy
T = temperature
To be ∆G negative, there should be decrease in enthalpy and increase in entropy of the system.
For many reactions entropy effects are small and it is the enthalpy that mainly determines whether the reaction can take place spontaneously. However, in certain types of reaction entropy is important and can dominate enthalpy. Some of the examples are:
- Generally, gases have higher entropy than liquids and solids because the molecules of gas have much more randomness. Liquids have higher entropy than that of solids but lower than that of gases. Hence, any reactions in which the reactants are solids and the products are liquids and gases are thermodynamically favored.
- When the number of products formed are more than that of reactants, degree of freedom increase and hence entropy also increases making the reaction thermodynamically favored. On the other hand, reactions in which the number of product molecules is less than the number of reactant molecules, entropy decreases and in such cases there must be significantly decrease in enthalpy to be ∆G negative.
- An open chain molecule has more entropy than a similar cyclic molecule because there are more conformations. Hence, ring opening reactions are thermodynamically favorable.
Kinetic requirements for a chemical reaction :
A negative ΔG is a necessary but not a sufficient condition for a reaction to occur spontaneously. For example, the reaction between H2 and O2 to give H2O has a large negative ΔG, but mixtures of H2 and O2 can be kept at room temperature for many centuries without reacting to any significant extent.
So, for a reaction to occur, the chemical reactions require some activation energy. Activation energy is defined as the minimum amount of extra energy required by a reacting molecule to get converted into product. It can also be described as the minimum amount of energy needed to activate or energize molecules or atoms so that they can undergo a chemical reaction or transformation.

In the above energy profile diagram, ∆Gf is the free energy of activation for forward reaction and ∆Gr is the free energy of activation for backward reaction.
Kinetically vs Thermodynamically controlled reaction
Q) Give labeled energy profile diagram illustrating kinetic versus thermodynamic control of the product.
Let us consider a chemical reaction in which a reactant ‘A’ gives two different products ‘B’ and ‘C’ by different mechanism.

In this figure, ‘C’ is thermodynamically more stable ( due to lower energy ) but ‘B’ is formed faster ( due to lower energy of activation).
If the reaction is irreversible, energy of activation is lower for the formation of B, so B is formed faster. So, the product ‘ B’ is said to be kinetically controlled.
However, if the reaction is permitted to approach equilibrium (i.e. if the reaction is reversible), the more stable product C predominates. Under these conditions, the product B that is first formed reverts to A. So, the product ‘C’ is said to be thermodynamically controlled.
Example of kinetic and thermodynamic control of the reaction :
Q) Explain the term kinetic and thermodynamic control of the reaction with suitable examples.
Q) Give the mechanism of kinetically controlled and thermodynamically controlled addition of HBr to 1,3-butadiene.
The kinetic and thermodynamic control of the reaction can be understood by taking an example of addition of HBr to 1,3-butadiene.
Addition of HBr to 1,3-butadiene gives two types of products- 1,2-addition product and 1,4-addition product.

Mechanism :
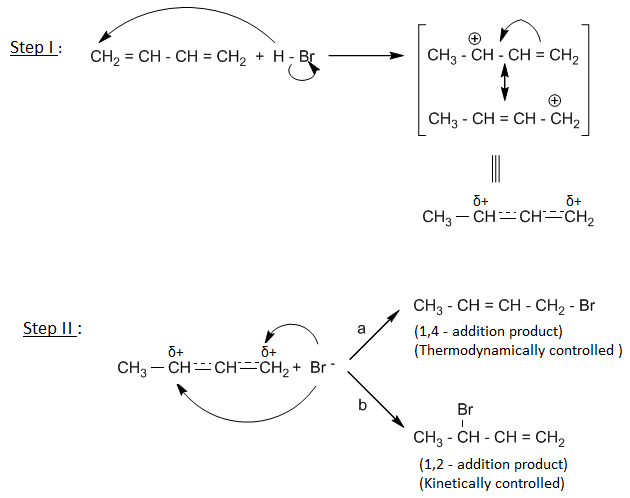
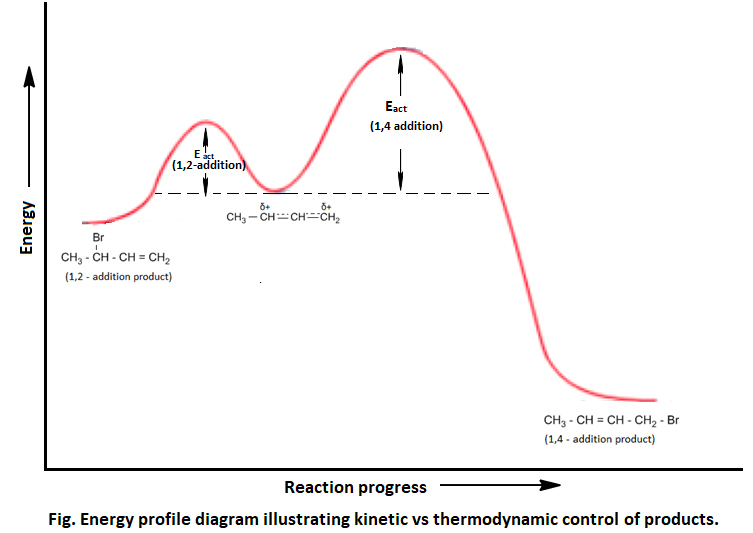
From the above data, it can be concluded that at – 800C, 1,2-product is formed faster than 1,4-product due to less energy of activation for 1,2-product. 1,2- addition product is formed as a major product. Thus 1,2-addition product is kinetically controlled product.
But when the temperature is raised the equilibrium is attended and the reaction becomes reversible. At this condition, the 1,4-addition product is formed as a major product because it is more stable (less energy) than 1,2-addition product. The 1,2-product is formed faster but it ionizes and converts into more stable 1,4-addition product. Thus 1,4- addition product is thermodynamically controlled.
Hammond postulate
Q) State Hammond postulate. Explain with a suitable example the application of Hammond postulate in determining the shape and geometry of transition state. How would you differentiate intermediate from transition state ?
Q) Give the statement of Hammond postulate. Why is it most useful ?
Transition states have zero life time, so it can not be isolated and studied directly and hence the information about their shape and geometries must be obtained from inference. Hammond postulate states that ‘ for any single reaction step, the geometry of the transition state for that step resembles to the compound (reactants or products) to which it is closer in free energy’.
Thus, for the endothermic reaction the transition state resembles the products more than the reactants because transition state is closer to product in energy profile diagram (i.e. ∆G2<∆G1).
For the exothermic reaction the transition state resembles the reactants more than the products because transition state is closer to reactant in energy profile diagram (i.e. ∆G1<∆G2).
 Hammond postulate is most useful in dealing with reactions with intermediates. For the reaction illustrated in energy profile diagram as below:
Hammond postulate is most useful in dealing with reactions with intermediates. For the reaction illustrated in energy profile diagram as below:
Reactant→T.S.1→Intermediate→T.S.2→Product

The T.S.1 lies much closer in free energy to the intermediate than to the reactants and hence we can predict that the geometry of T.S.1 resembles that of intermediate more than that of reactants. Similarly, T.S.2 also has a free energy much closer to that of the intermediate than to the products. Therefore, both transition states resemble the intermediate more than the reactants and products.
Difference between transition states and intermediates :
The transition state refers to an imaginary molecule having zero life time and cannot be isolated. In this state the system possesses maximum energy and is most unstable. It is impossible to observe them directly and information about their geometries must be obtained from inference.
On the other hand, the relatively stable products formed from the reactants during reaction that further reacts to give the final product is called intermediate. The energy of intermediate is less than that of transition state and can be isolated and studied. Their geometries can be obtained by using many techniques such as IR, NMR spectroscopy, etc. We often use the knowledge of intermediates to determine the shape and geometry of transition states.
Q) State Hammond postulate. What information does the postulate provides about the structure of T.S. in the following energy profile diagram ?

Microscopic reversibility
Under the same reaction condition, if the reaction is reversible, the forward and reverse reactions must proceed by same mechanism. This is called the principle of microscopic reversibility. For example, if in a reaction A → B there is an intermediate ‘C’, then ‘C’ must also be an intermediate in the reaction B → A.

This is a very useful principle to predict the mechanism of reversible reaction.
For example, when alcohol is treated with H2O and H2SO4, alkene is obtained via carbocation intermediate. When same alkene is treated with H2O and H2SO4 , alcohol is obtained via same carbocation intermediate.

Methods of determining reaction mechanism
There are a number of commonly used methods for determining reaction mechanism. In most cases, one method is not sufficient. Reaction intermediates are the important class of chemical species, which are quite helpful in understanding the mechanism of a chemical reaction.
1. Isolation of intermediate :
Isolation of intermediates gives the valuable information to identify the exact mechanism. It is sometimes possible to isolate an intermediate from a reaction mixture by stopping the reaction after a short time or by the use of very mild conditions. For example, Hoffmann’s bromamide reaction:

During this reaction following intermediates were formed and isolated:

In order to explain the formation of these intermediates, following mechanism was proposed:
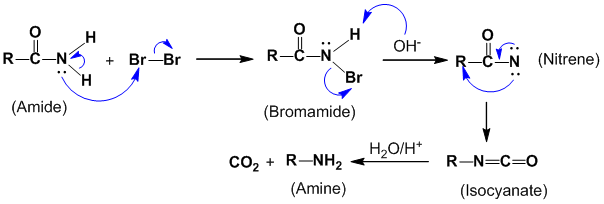
2. Detection of intermediate :
In many cases, intermediate cannot be isolated but can be detected. It can be detected by IR, NMR or other spectroscopic technique. The detection of aman spectra of NO2+ (nitronium ion) indicates it is an intermediate in nitration of benzene. Hence, nitration of benzene is electrophilic substitution reaction and the following mechanism for the nitration of benzene was proposed:

3. Stereochemical evidence :
If the products of a reaction are capable of existing in more than one stereoisomeric forms, the form which is obtained may give information about the mechanism. For example, in SN2 reaction the product product obtained is always inverted. This is only possible if the attack of nucleophile and removal of leaving group takes place simultaneously. In such cases, nucleophile attacks from backside of leaving group to give inverted product.

4. Identification of products :
Identification of the products of a reaction also helps to define the reaction mechanism.
For example, elimination reaction of 2-bromobutane.

From the identification of but-2-ene as major product and bu-1-ene as minor product, following mechanism can be proposed:

5. Isotope labeling ( Tracer technique) :
Q) What is the scope of isotope labeling (tracer technique) in the determination of reaction mechanism? Explain with suitable example.
When one isotope of the bonded atom is replaced by its heavier isotope, the rate of bond breaking becomes slower. So by comparing the rate of original bond breaking with that of the heavier isotope substituted bond, we can determine whether a particular bond breaks in the rate determining step or not. The effect of heavier isotope on the rate of the bond breaking is called isotope effect.
Much important information can be obtained by using molecules that have isotopically labeled and tracing the path of the reaction in that way.
For example, ester undergoes hydrolysis to form a mixture of carboxylic acid and alcohol.

The products may have been formed by acyl-oxygen bond fission (a) or by alkyl-oxygen bond fission (b). If the hydrolysis of ester is carried out using H2O18 (i.e. oxygen having mass 18), the following products would be expected:

In this reaction, the appearance of O18 in carboxylic acid has been confirmed by mass spectroscopy. So the fission must have occurred at acyl-oxygen bond in the hydrolysis of ester.
Q) Show that hydrolysis of ethyl acetate occurs acyl-oxygen bond fision but not ethyl-oxygen bond fission with the help of tracer technique.
6. Trapping of intermediate :
In some cases, the suspected intermediate is known to be one that reacts in a given way with a certain compound. The intermediate can then be trapped by running the reaction in the presence of that compound.
For example, benzyne reacts with dienes in Diels- Alder reaction. In any reaction where a benzyne is suspected intermediate, the addition of a diene and the detection of Diels-Alder addition product indicates that the benzyne was present.

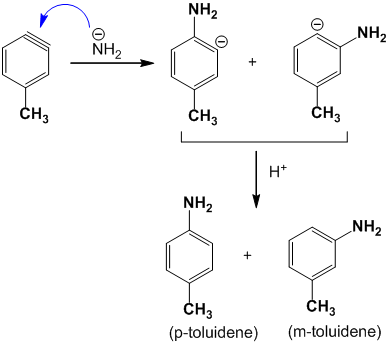
Q) The action of p-chlorotoluene with sodamide in presence of liquid ammonia yields a mixture of para and meta toluidene. Give the mechanism of the reaction.
Q) Using the method of trapping intermediate, predict the suitable mechanism for the following reaction:

Answer :
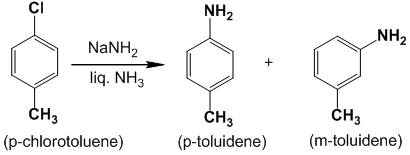
The mechanism of this reaction is believed to proceed as follows by trapping benzyne intermediate.
Step – I : The first step involves the loss of H+ and Cl- from p-chlorotoluene to form a benzyne intermediate.

Step – II : The second step involves the attack of the benzyne intermediate by NH2– followed by protonation.
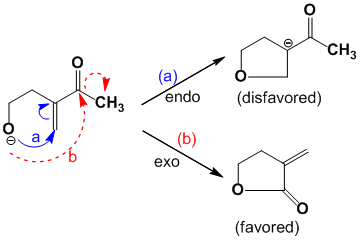
Baldwin’s rule of ring closure
J.E Waldwin has proposed a set of rules for ring closure reactions of 3 to 7 membered rings. The process could be ‘endo’ and ‘exo’. ‘Exo’ means the bond broken during the ring closure is outside while the ‘endo’ means the bond broken during the ring closure is inside.

The hybridization of carbon atom (C) undergoing the ring closure reaction is of three types :
Tetrahedra (Tet) – sp3 (i.e. a single bond centre)
Triginal (Trig) – sp2 (i.e. a double bond centre)
Diagonal (Dig) – sp (i.e. a triple bond centre)
The following are Baldwin’s rule for closing rings of 3 to 7 members:
Rule 1 : Tetrahedral system
- 3 to 7 – Exo – Tet are all favored
- 5 to 6 – Endo – Tet are disfavored
Rule 2 : Trigonal system
- 3 to 7 – Exo – Trig are favored
- 3 to 5 – Endo – Trig are disfavored
- 6 to 7 – Endo – Trig are favored
Rule 3 : Diagonal system
- 3 to 4 – Dig are disfavored
- 5 to 7 – Dig are favored
- 3 to 7 – Dig are favored
Disfavored does not mean it cannot occur but only it is more difficult than the favored cases. A reaction that is disfavored (slow) does not have a rate that is able to compete effectively with an alternative reaction that is favored (fast).
Example: 5 – Exo – Trig
References
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- March, j., Advanced Organic Chemistry, Fourth edition, Wiley Eastern Ltd. India, 2005.
- http://epgp.inflibnet.ac.in/epgpdata/uploads/epgp_content/chemistry/05.organic_chemistry-ii/03.thermodynamic_and_kinetic_requirements_of_a_reaction/et/5547_et_et.pdf
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Equilibria/Chemical_Equilibria/Principles_of_Chemical_Equilibria/Kinetically_vs_Thermodynamically_Stable
- https://www.mt.com/my/en/home/applications/L1_AutoChem_Applications/L2_ReactionAnalysis/reaction-mechanisms.html
- https://www.intechopen.com/books/chemometrics-in-practical-applications/analysis-of-chemical-processes-determination-of-the-reaction-mechanism-and-fitting-of-equilibrium-an



