Contents
Development of organic chemistry

Around 1780 chemists begin to distinguish chemical substances into two categories on the basis of source from which they were derived.
- Organic compounds : Obtained from organism( i.e. plants or animals). Eg. glucose, protein, nucleic acid, etc.
- Inorganic compounds : Prepared from non-living sources ( minerals). Eg. NaCl, SiO2, etc.

In 1815, Berzelius (Swedish chemist) proposed vital force theory.
Vital force theory
According to the vital force theory, a vital/mysterious force present inside the living organism was responsible for the formation of organic compounds, so organic compounds can not be prepared in lab.
- Later, in 1828, German chemist, Friedrich Wohler obtained urea accidently in lab from ammonium cyanate ( by rearrangement).
- Wohler was in fact preparing ammonium cyanate by heating potassium cyanate and ammonium chloride.
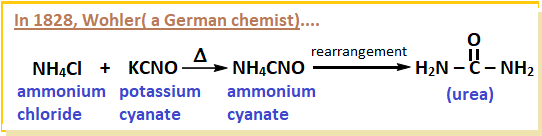
- This was a milestone in organic chemistry.
- This preparation broke down the old concept i.e. vital force theory .
- The second organic compound synthesized in lab was acetic acid by Kolbe from it’s respective elements C, H and O.
- Third organic compound synthesized in lab was methane ( marsh gas) by Berthelot.
- Now more than 5 million organic compounds are known and hundreds of new organic compounds are being discovered daily.
Organic chemistry covers broad area :
Petrol, diesel, medicines, clothes, colours, DNA, enzymes, etc. are nothing but are organic compounds.
So, it is compulsory to know about organic chemistry to understand how does petrol/diesel produces energy, how does a medicine works, etc.
Modern definition of organic compounds
Organic compounds are defined as the hydrocarbons ( compounds containing carbon and hydrogen) and their derivatives in which covalently bonded carbon is an essential constituent.

Organic chemistry
Organic chemistry is the branch of chemistry which deals with the study of hydrocarbons and their derivatives.
Differences between organic and inorganic compounds
| Organic compounds | Inorganic compounds |
|
|
|
|
|
|
|
|
|
|
Eg. C2H5OH and CH3-O-CH3 are isomers. |
|
Some of the unique properties of carbon
(Reasons for separate study of organic compounds) :
1. Tetracovalency of carbon :
The ground state electronic configuration of carbon is :

But it undergoes excited state during combination with other elements. In excited state, one of the paired electrons from 2s orbital gets promoted to vacant 2pz orbital to form four unpaired electrons in the valence shell.

This atom acquires octet by sharing four electrons with other atoms during compound formation and thus shows tetracovalency. Eg.

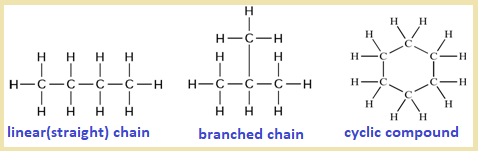
2. Catenation property :
The process of forming covalent bonds with atoms of the same element to give macromolecules or polymers ( long-chain) is called catenation property. It is one of the remarkable properties of carbon atom by which carbon atoms can link with each other to form either linear (straight) chain or branched chain or cyclic compounds. Eg.
3. Capacity to form multiple bonds :
Carbon atom can form double or triple bonds with other elements like carbon, nitrogen, oxygen, etc. Eg.

The above mentioned unique properties of carbon have been able to form large number of organic compounds.
See the Classification of Organic compounds……
Objective questions and their answers
1) Vital force theory was first discarded by:
a. Berzelius b. Wohler
c. Kolbe d. Berthelot
2) The first organic compound synthesized in lab was
a. Urea b. Methane
c. Acetic acid d. Ethane
3) The first organic compound synthesized in lab from its elements was
a. Urea b. Methane
c. Acetic acid d. Ethane
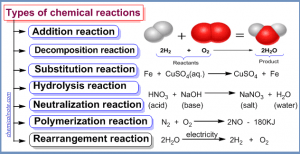
4 ) NH4CNO → NH2CONH2 . This reaction is:
a. Addition reaction
b. Elimination reaction
c. Rearrangement reaction
d. Substitution reaction
5) ……… is often called as ‘king of element’.
a. H b. C
c. N d. O
6) Carbon always forms …….covalent bonds.
a. 2 b. 3
c. 4 d. 5
7) Organic compounds are very large in number. This is due to:
a. Small size of carbon
b. Catenation property of carbon
c. Valency of carbon
d. All of the above
Answer … 1-b( discarded- dismissed, rejected), 2-a, 3-c, 4-c, 5-b, 6-c, 7-b.
References
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- Sthapit, M.K., Pradhananga, R.R., Foundations of Chemistry, Vol 1 and 2, Fourth edition, Taleju Prakashan, 2005.
- https://www.sciencedirect.com/topics/earth-and-planetary-sciences/organic-chemistry
- https://chemistry.stanford.edu/research/research-areas/organic-chemistry