Contents
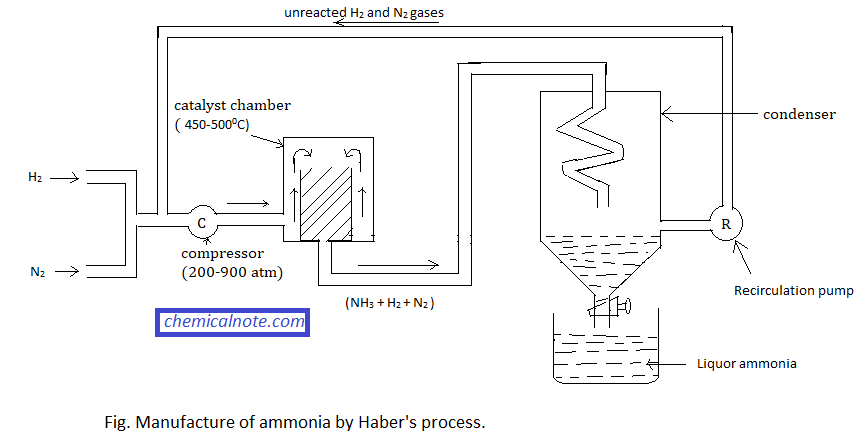
Manufacture of ammonia by Haber’s process:
When a mixture of nitrogen and hydrogen gas in the ratio 1:3 by volume is heated at a temperature of 450-5000C and 200-900 atmospheric pressure in the presence of iron as catalyst and molybdenum as promoter, ammonia gas is produced.
- Low temperature : Since the reaction is exothermic, low temperature is favourable for the formation of ammonia. If the temperature is below 4500C, N2 and H2 react too slowly to attain equilibrium state. Thus an optimum temperature of about 450-5000C is maintained.
- High pressure : The total volume of product is lower than total volume of reactant so, equilibrium shifts in forward direction with the increase in pressure. In practice, 200 to 900 atmospheric pressure is applied.
- High concentration of reactants : Since reaction proceeds in forward direction with decrease in volume, so either one or both reactants( H2 and N2) should be used in excess amount for high yield of NH3.
- Catalyst : Presence of the catalyst speeds up the rate of reaction. Finely divided Iron catalyst and Molybdenum promoter is used.
- Purity of hydrogen and nitrogen : The nitrogen and hydrogen gas should be very pure, otherwise the catalyst is poisoned and decreases catalytic activity.
Physical Properties of Ammonia :
- It is a colourless and pungent smelling gas.
- It is lighter than air.
- It is easily liquefiable gas. It can also be solidified. Melting point of solid ammonia is -780C and boiling point of liquid ammonia is -33.40C.
- It is neither combustible nor a supporter of combustion.
- It is highly soluble in water as it forms intermolecular hydrogen bond with water.
Chemical Properties (reactions) of Ammonia :
- Basic nature:
- Ammonia changes the colour of moist litmus paper into blue.
- It dissolves in water to give OH– ions.
- It reacts with acid to give salt.
- Due to presence of lone pair of electrons on the nitrogen atom, ammonia acts as a Lewis base,
- Action with oxygen : When ammonia is heated with oxygen, it gets oxidized to nitrogen gas. Here ammonia acts as reducing agent.
- Action with metal oxides : when ammonia gas is passed over heated copper oxide or lead oxide, metal oxide is reduced to respective metals.
- Action with bleaching powder : When ammonia gas is heated with bleaching powder, it reduces CaOCl2 into CaCl2.
- Action with fluorine :
- Action with chlorine :
- Action with bromine :
- Action with iodine :
- Action with copper sulphate solution :
Uses of ammonia :
- It is used for the manufacture of urea.
- It is used as cooling agent in refrigerator.
- It is used for reducing metal oxide.
- It is used to manufacture nitric acid by Ostwald’s process
Structure of ammonia :
REFERENCES :
- Agrawal, S. K., Lal, K., Advanced Inorganic Chemistry, Fifth Revised Edition, Pragati Prakashan, Meerut, 2001.
- Cotton, F. A., and Wilkinson, G., Advanced Inorganic Chemistry, Fifth edition, John Wily and Sons, Singapore, 1995.
- Day, C.M., Selbin, J., Theoritical inorganic Chemistry, second edition, Affiliated East-West Press Pvt. Ltd., New Delhi, 2002.
- Lee, j. D., Concise Inorganic Chemistry, Fifth Edition, Joh, Wiley and Sons, Inc., 2007.
- Sarkar, R., General and Inorganic Chemistry, Second Edition, New Central Book Agency(P) Ltd., India, 2007.
- Shriver, D. F., Atkins, P. W., Inorganic Chemistry, Fifth Edition, Oxford university Press, 2010.
- Mitra, L.A. , A Text Book of Inorganic Chemistry, Ghos and Company, 61st edition, 1996.
- https://www.aiche.org/resources/publications/cep/2016/september/introduction-ammonia-production
- https://pubchem.ncbi.nlm.nih.gov/compound/Ammonia
- https://en.wikipedia.org/wiki/Ammonia